Watch television lately, peruse a social media outlet or simply pay attention to any billboards along a highway and you will no doubt see notification of a “MEDICAL “RECALL” – (with certainly information on who to contact if you were affected). This is not that kind of article. Instead, this article will examine what it means when a medical device is recalled, the FDA classification of such, and the likely litigation course that will follow. The hope is that such information will help impacted patients navigate the landscape by educating themselves as to what it all means.
MEDICAL DEVICE RECALL

Medical devices as an industry are certainly no stranger to the recall process. When it does occur, recall efforts require speed to ensure patient safety. The decision to pull a defective medical product can often be a quick one. Though the system utilized to notify patients, however, is usually not.
Reliance on snail mail, paper communications, and other intermediaries can often take weeks to notify a patient that a medical device implanted in their body is defective. Critics often point to the lack of uniformity in the notification process with varying differences by the company issuing the recall, the hospital addressing the recall, or the specific product at issue.
THE FDA
The FDA plays an important role in the recall effort – yet it can often add to the notification delays, by taking 90 days or more to first categorize the medical device recall. Yes, the FDA receives thousands of recalls every year and are tasked with labelling the recall either a Class I, II, or III event. Class I event are the most serious – signifying a reasonable probability that the use of or exposure to such a violative product will cause serious adverse health consequences or death.


So now you have been notified that a product implanted in your body is defective and can cause serious adverse health consequences. What now? The simple answer is to go see you doctor as soon as possible to discuss your treatment options.
With medical recall cases, your doctor is likely your best ally because they too are often victims in this process – usually learning at the same time as their patients of the defective nature of the medical device they utilized.
MDL
In recent years, many of these medical device recalls have led to litigation against the medical product manufacturers. Medical product manufacturers of CPAP devices; Hernia Mesh; Surgical Staplers; and most recently Exactech knee replacement devices have all faced litigation for their respective defective medical products. The frequency of such recalls and resulting litigation has led Congress to intervene with the creation of the Multidistrict Litigation (“MDL”) process. When civil lawsuits are filed in federal courthouses across the country due to a medical device recall, they are often consolidated and transferred to a single federal court as an MDL.
Congress created MDLs to save time and money and to ensure similar outcomes in lawsuits that involve large numbers of people and contain similar allegations.
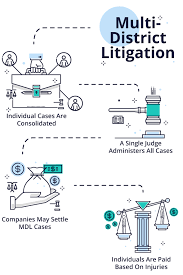
MDLs often center on defective medical devices, dangerous prescription drugs, and securities fraud. They sometimes involve thousands of individual lawsuits or dozens of class actions. The MDL pretrial proceedings generally guide:
- Gathering evidence during the discovery process,
- Motions to dismiss the case,
- Motions for summary judgment,
- Evidentiary challenges,
- Depositions and interrogatories,
- Settlement offers.
If the case does not settle during these MDL pretrial proceedings, it is then transferred back to the district court from which it originated for the trial.
now WHAT?
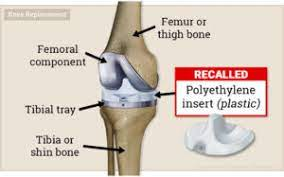
Though the special MDL proceedings aide in collectively proving what a medical product manufacturer knew about their defective product before they took it to market to be utilized by doctors, it may not always be the best mechanism for a patient to prove his or her individualized damages. Your recovery is just that – yours. All too often, patients seek counsel who are skilled at amassing large numbers of claims to file but know very little about the medicine involved and lack the experience to present such a case to a jury.
Seek counsel who is not only competent regarding the medical device recalled, but also the MEDICINE surrounding the device usage and implantation process. Your ultimate recovery is likely dependent upon it.
By
Michael S. Sepcich
Davida Packer, MD
Contact our office today to see how we can best assist you.


